BACKGROUND: Application specifications for ISBT 128 bar code symbology and the International Council for Commonality in Blood Bank Automation (ICCBBA) were created in 1994. By June 2000, the FDA considered ISBT 128 a standard for uniform labeling of blood and blood components. Our blood center initiated a change process for ISBT 128 implementation and “went live” in 2003.STUDY DESIGN AND METHODS: The intention to adopt ISBT 128 symbology with hospitals was actively communicated in October 2001. A Codabar‐ISBT label cross‐reference book was developed, FDA approval for the fullface label format in April 2002 was requested, and FDA approval was received in March 2003. In December 2002, donor identification labels and number sets were ordered, and an integration test plan was subsequently developed with departmental process flowcharts for each of the nine affected departments. Each step was tested, the labeling changes were approved in May 2003, training was completed in June 2003, and ISBT bar code symbology was implemented on July 1, 2003. A written survey was sent to hospital transfusion services in April 2004.RESULTS: Implementation went smoothly except for an unanticipated high rate of “no‐reads” on some analyzers in the testing lab.
Search the history of over 357 billion web pages on the Internet.

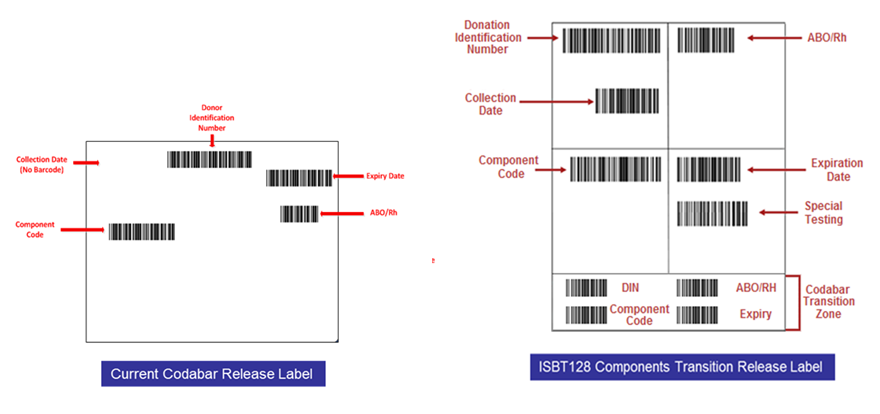
The hospitals spent an average of 18 hours preparing for changes, 14 hours on validation, 4 hours on documentation and procedure development, and 8 hours on training.CONCLUSION: ISBT bar code symbology was successfully implemented. Hospital transfusion services made some adjustments and, overall, readily accepted the new bar code symbology.
The publication of new standards for terminology and labeling marks an important step in ensuring consistency and traceability of cellular therapies at the global level. However, it is only with the widespread implementation of the standard that the benefits can be truly realized.
Isbt 128 Barcode
This paper provides guidance on the practical aspects of adopting these new standards for organizations with differing current levels of computerization. It discusses project management, equipment, licensing, and validation topics. Abstract = 'The publication of new standards for terminology and labeling marks an important step in ensuring consistency and traceability of cellular therapies at the global level.
Isbt 128 Labeling System

Isbt 128 Product Codes
However, it is only with the widespread implementation of the standard that the benefits can be truly realized. This paper provides guidance on the practical aspects of adopting these new standards for organizations with differing current levels of computerization. It discusses project management, equipment, licensing, and validation topics.'
Do note, however, that you will need a Nintendo 64 emulator. Kaze Emanuar, the talented man behind some incredible projects such as, and, has released a new fan-game that aims to bring together the characters of Super Mario 64 with the storyline and the world of Ocarina of Time.As with all fan-games, this is completely free and anyone can download and play it. You will also need to enable 8MB ram in the emulators settings or it will only give you a black screen.Kaze Emanuar also plans to give Majoras Mask the same Super Mario 64 treatment, and he has some crazy ideas for the mask.  Of course this will happen if there is a demand and if a lot of people download his Super Mario 64: Ocarina of Time project.Those interested can download it from.Have fun!
Of course this will happen if there is a demand and if a lot of people download his Super Mario 64: Ocarina of Time project.Those interested can download it from.Have fun!
TY - JOURT1 - ISBT 128 implementation plan for cellular therapy productsAU - Ashford, PaulAU - Distler, PatAU - Gee, AdrianAU - Lankester, AlanAU - Larsson, StellaAU - Feller, IreneAU - Loper, KathyAU - Pamphilon, DerwoodAU - Poston, LeighAU - Rabe, FranAU - Slaper-Cortenbach, InekeAU - Szczepiorkowski, ZbigniewAU - Warkentin, Phyllis IrenePY - 2007Y1 - 2007N2 - The publication of new standards for terminology and labeling marks an important step in ensuring consistency and traceability of cellular therapies at the global level. However, it is only with the widespread implementation of the standard that the benefits can be truly realized. This paper provides guidance on the practical aspects of adopting these new standards for organizations with differing current levels of computerization. It discusses project management, equipment, licensing, and validation topics.AB - The publication of new standards for terminology and labeling marks an important step in ensuring consistency and traceability of cellular therapies at the global level. However, it is only with the widespread implementation of the standard that the benefits can be truly realized. This paper provides guidance on the practical aspects of adopting these new standards for organizations with differing current levels of computerization. It discusses project management, equipment, licensing, and validation topics.KW - CodingKW - ImplementationKW - ISBT 128KW - LabelingKW - TerminologyUR -10.1002/jca.20146DO - 10.1002/jca.20146M3 - ArticleVL - 22SP - 258EP - 264JO - Journal of Clinical ApheresisJF - Journal of Clinical ApheresisSN - 0733-2459IS - 5ER.